Brief Summary
This video explains the concept of the mole and its importance in chemistry. The mole is a unit of measurement that represents a specific number of particles, such as atoms or molecules. This number is known as Avogadro's constant, which is approximately 6.02 x 10^23. The video explains how to use the mole to calculate the number of atoms or molecules in a given amount of substance.
- The mole is a unit of measurement that represents a specific number of particles.
- Avogadro's constant is 6.02 x 10^23, which is the number of particles in one mole.
- The mole is used to calculate the number of atoms or molecules in a given amount of substance.
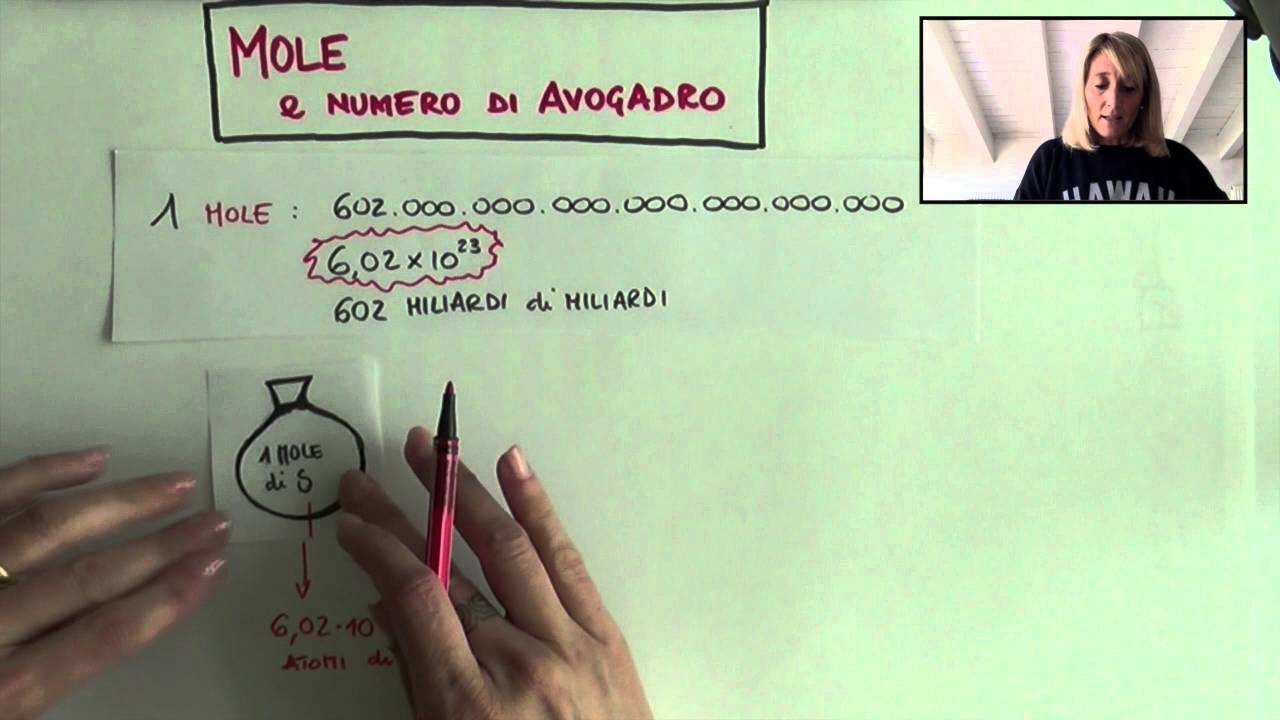
What is a Mole?
The video begins by introducing the concept of the mole. The mole is a unit of measurement that represents a specific number of particles, such as atoms or molecules. This number is known as Avogadro's constant, which is approximately 6.02 x 10^23. The video explains that the mole is similar to other units of measurement, such as a dozen or a pair, which represent a specific number of items.
Why is the Mole Important in Chemistry?
The video then explains why the mole is important in chemistry. The mole is a useful unit of measurement because it allows chemists to work with large numbers of atoms and molecules. The video explains that it is difficult to measure the mass of individual atoms and molecules, so chemists use the mole to represent a specific number of these particles.
Calculating the Number of Atoms or Molecules
The video concludes by providing examples of how to calculate the number of atoms or molecules in a given amount of substance. The video explains that to calculate the number of atoms or molecules in a given amount of substance, you need to multiply the number of moles by Avogadro's constant. The video provides examples of how to calculate the number of atoms in a given amount of iron and the number of molecules in a given amount of carbon dioxide.