Brief Summary
This video provides a comprehensive guide on drawing Lewis structures for various molecules, organic compounds, and polyatomic ions. It covers essential concepts such as valence electrons, bond formation, formal charge calculation, resonance structures, molecular geometry, polarity, and hybridization. The video also introduces the "multiple of eight" rule as a helpful technique for drawing complex Lewis structures.
- Understanding valence electrons and bond formation
- Calculating formal charges and identifying resonance structures
- Determining molecular geometry and polarity
- Applying the "multiple of eight" rule for complex structures
Basics of Lewis Structures
The video starts with the basics of the periodic table, focusing on the second-row elements like boron, carbon, nitrogen, oxygen, and fluorine, and their valence electrons. It explains how these elements tend to form a specific number of bonds to achieve a stable octet. Non-metals in the upper right corner of the periodic table tend to acquire electrons, while metals on the left side tend to give them away. Elements in the third row, such as phosphorus, sulfur, and chlorine, can have expanded octets due to the availability of 3D orbitals. The number of bonds an element forms can indicate its formal charge, with deviations from the ideal number often resulting in a formal charge.
Drawing Lewis Structures for Diatomic Molecules
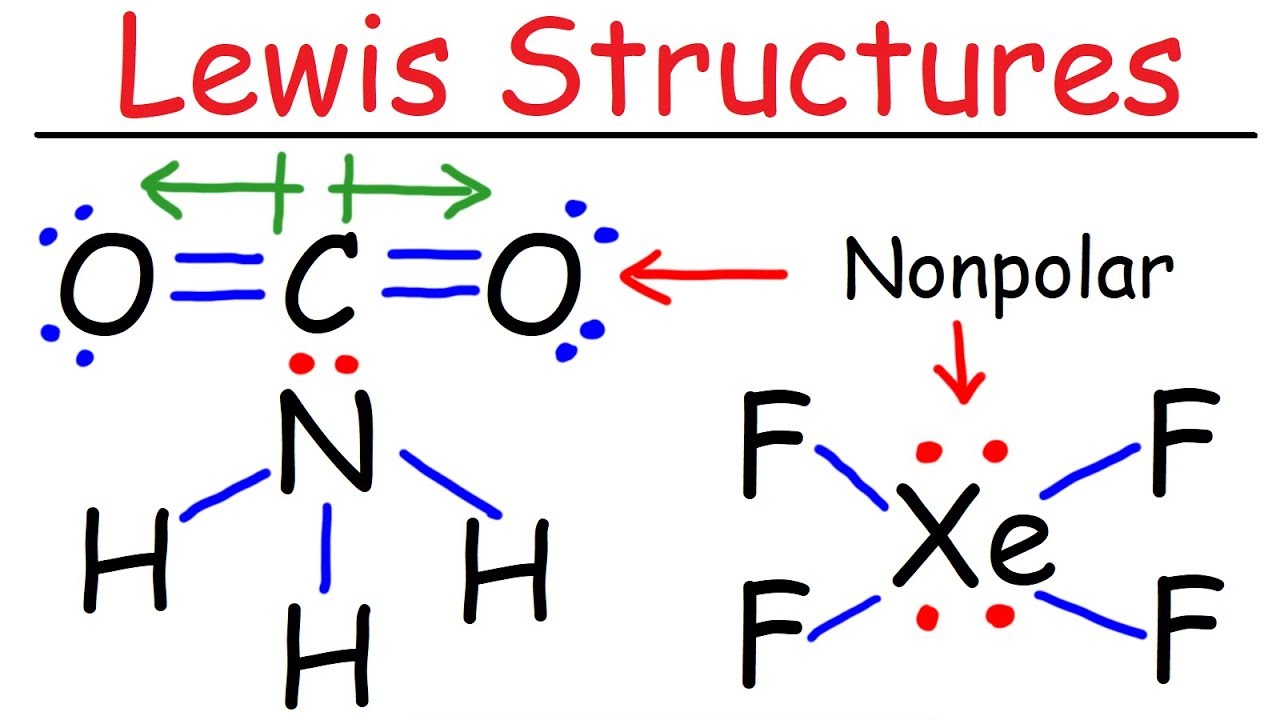
The video explains how to draw Lewis structures for diatomic molecules like fluorine (F2), oxygen (O2), and nitrogen (N2). The first step is to add up the total number of valence electrons in the molecule. Then, based on the number of valence electrons, determine the number of bonds between the atoms. For example, fluorine forms a single bond, oxygen forms a double bond, and nitrogen forms a triple bond. Add lone pairs to the atoms so that each atom has an octet of electrons. The video also discusses the polarity and bond order of these molecules.
Lewis Structures, Molecular Geometry, and Polarity
The video explains how to draw Lewis structures for molecules like hydrogen gas (H2) and borane (BH3). Hydrogen forms a single bond and does not have lone pairs. Boron has an incomplete octet and forms three bonds in BH3. The video also discusses the molecular geometry and polarity of BH3, which is trigonal planar and non-polar. It is recommended to use Google Images and search for "molecular geometry worksheet" to familiarize yourself with the different shapes and names of those shapes.
Formal Charge and Molecular Geometry of Water, Hydronium, and Hydroxide
The video explains how to draw Lewis structures for water (H2O), hydronium ion (H3O+), and hydroxide ion (OH-). It also explains how to calculate the formal charge of an atom in a molecule using the equation: formal charge = valence electrons - bonds - dots. The video also discusses the molecular geometry and electron pair geometry of these molecules. Water has a bent molecular geometry and a tetrahedral electron pair geometry. Hydronium ion has a trigonal pyramidal molecular geometry and a tetrahedral electron pair geometry. Hydroxide ion has a linear molecular geometry.
Polar vs Nonpolar Molecules
The video explains why carbon dioxide (CO2) is nonpolar while carbon monoxide (CO) is polar, even though both molecules contain polar bonds. The video analyzes the electronegativity difference between carbon and oxygen, which makes the C-O bond polar. In CO2, the dipole moments cancel each other out due to the symmetry of the molecule, resulting in a net dipole moment of zero. In CO, the dipole moments do not cancel, resulting in a net dipole moment and a polar molecule.
Hybridization
The video explains how to determine the hybridization of the central atom in a molecule based on the number of groups (atoms and lone pairs) attached to it. If the number of groups is two, the hybridization is sp; if it is three, the hybridization is sp2; and if it is four, the hybridization is sp3. The video provides examples of determining the hybridization of boron in BH3 and oxygen in water.
Lewis Structure, Molecular Geometry, Polarity, and Hybridization of Methane and Ammonia
The video explains how to draw the Lewis structure for methane (CH4), determine its molecular geometry (tetrahedral), polarity (nonpolar), and hybridization (sp3). It also explains how to draw the Lewis structure for ammonia (NH3), determine its molecular geometry (trigonal pyramidal), polarity (polar), and hybridization (sp3).
Polarity of Carbon Dioxide and Sulfur Dioxide
The video explains why carbon dioxide (CO2) is nonpolar while sulfur dioxide (SO2) is polar. CO2 has a linear molecular geometry, and the dipole moments cancel each other out. SO2 has a bent molecular geometry due to the presence of a lone pair on the central sulfur atom, and the dipole moments do not cancel.
Sulfur Bonding
The video discusses the bonding behavior of sulfur, which can form two to six bonds depending on the electronegativity of the atoms it is bonded to. In hydrogen sulfide (H2S), sulfur forms two bonds and has a partial negative charge. In sulfur hexafluoride (SF6), sulfur forms six bonds and is nonpolar due to the symmetry of the molecule.
Molecular Geometry and Hybridization of Phosphorus Pentachloride and Sulfur Hexafluoride
The video explains how to draw the Lewis structure for phosphorus pentachloride (PCl5), determine its hybridization (sp3d), molecular geometry (trigonal bipyramidal), and polarity (nonpolar). It also explains the bond angles in the trigonal bipyramidal shape. The video also discusses the hybridization of sulfur hexafluoride (SF6), which is sp3d2.
Multiple of Eight Rule
The video introduces the "multiple of eight" rule as a helpful technique for drawing complex Lewis structures. This rule states that if a molecule has a multiple of eight valence electrons and no hydrogen atoms, the central atom will have no lone pairs. The video provides examples of applying this rule to sulfur tetrafluoride (SF4), sulfur difluoride (SF2), and xenon tetrafluoride (XeF4).
Molecular Geometry and Polarity of Iodine Pentafluoride and Triiodide Ion
The video explains how to draw the Lewis structure for iodine pentafluoride (IF5), determine its molecular geometry (square pyramidal), and polarity (polar). It also explains how to draw the Lewis structure for the triiodide ion (I3-), determine its molecular geometry (linear), and formal charges.
Stable Lewis Structures
The video discusses how to identify the most stable Lewis structure when there are multiple acceptable Lewis structures. The most stable Lewis structure is the one where the formal charge of the central element is zero. The video provides examples of drawing resonance structures for the carbonate ion (CO32-) and the nitrite ion (NO2-).
Resonance Structures
The video explains how to draw resonance structures for molecules like BF3. Boron has an incomplete octet, but it can form a double bond with fluorine to satisfy its octet. However, the resonance structure with the double bond is less stable because it has formal charges.
Lewis Structures for Sulfur Dioxide and Nitrate Ion
The video discusses the Lewis structures for sulfur dioxide (SO2) and the nitrate ion (NO3-). It explains how to draw the most stable Lewis structure for SO2, where the formal charge of the sulfur atom is zero. It also explains how to draw resonance structures for the nitrate ion.
Drawing Lewis Structures for Complex Molecules
The video provides guidance on drawing Lewis structures for complex molecules with multiple elements, such as SOF2 and POCl3. It emphasizes the importance of considering the bonding preferences of each element and using the "multiple of eight" rule to determine the number of lone pairs on the central atom.
Lewis Structures for Polyatomic Ions
The video explains how to draw Lewis structures for polyatomic ions, such as sulfate (SO42-) and phosphate (PO43-). It also challenges the viewer to draw the Lewis structures for the perchlorate ion (ClO4-), chlorate ion (ClO3-), chlorite ion (ClO2-), and hypochlorite ion (ClO-).
Lewis Structures for Radicals
The video explains how to draw Lewis structures for radicals, which are substances with an odd number of electrons. The video provides examples of drawing Lewis structures for nitrogen dioxide (NO2) and nitrogen monoxide (NO).
Lewis Structure for Thiocyanate Ion
The video explains how to draw the Lewis structure for the thiocyanate ion (SCN-), a polyatomic ion with three different elements. It discusses the importance of considering the electronegativity and size of the atoms when determining the most stable resonance form.
Lewis Structures for Organic Molecules
The video explains how to draw Lewis structures for organic molecules, such as ethane (C2H6), ethene (C2H4), ethyne (C2H2), methanol (CH3OH), ethanal (CH3CHO), propanone (CH3COCH3), dimethyl ether (CH3OCH3), ethanoic acid (CH3COOH), methyl ethanoate (CH3COOCH3), ethylamine (CH3CH2NH2), ethanamide (CH3CONH2), and ethanenitrile (CH3CN). It emphasizes the importance of remembering the bonding preferences of carbon, hydrogen, oxygen, and nitrogen.