Brief Summary
This video explains the basics of drug-drug interactions, focusing on the mechanisms of CYP450-mediated inhibition. It covers active and allosteric sites, binding affinity (Km and Vmax), intrinsic clearance, and different types of inhibition: competitive, non-competitive, mixed, and irreversible. Understanding these mechanisms is crucial for preventing adverse drug events.
- Drug-drug interactions occur when one drug alters the disposition of another.
- CYP450 enzymes are key players in drug metabolism and interactions.
- Inhibition can be reversible (competitive, non-competitive) or irreversible.
- Binding affinity (Km), intrinsic clearance (Vmax/Km) are important concepts.
Intro
Multiple drugs are often used in clinical practice due to patients presenting with multiple chronic diseases, and several medications are frequently administered concurrently to improve drug adherence. However, administering multiple drugs simultaneously increases the risk of drug-drug interactions, potentially leading to adverse drug events. Drug-drug interactions occur when a perpetrator drug alters the disposition of a victim drug. The most common mechanism is the inhibition of cytochrome P450 (CYP450) enzymes, primarily in the liver and gut mucosa, which are crucial for clearing compounds from the bloodstream. CYP450 inhibition can be reversible (competitive and non-competitive) or irreversible (mechanism-based), each requiring a unique clinical management strategy.
Active Site and Allosteric Site
The active site is a specific region within an enzyme where a molecule binds and a catalytic reaction occurs to convert a metabolite. The allosteric site is a separate region on the enzyme that allows molecules to influence enzyme activity, acting as either activators or inhibitors. Drugs binding to the allosteric site can alter the enzyme's three-dimensional structure, preventing substrate binding or catalysis. Non-competitive inhibition is often caused by allosteric regulation. Substrates are drugs that bind to the active site and are transformed into metabolites.
Binding Affinity
Binding affinity refers to the strength of attraction between an enzyme and its substrate, varying based on the substrate's chemical structure and physical properties. Substrates are classified as weak, intermediate, or strong based on their affinity for a specific enzyme. Binding affinity is measured by the Michaelis constant (Km), which represents the substrate concentration at which 50% of the maximum metabolic reaction rate (Vmax) is achieved. Vmax is the maximum speed of product creation for a given amount of enzyme. A small Km indicates strong binding, meaning Vmax is reached at lower substrate concentrations, while a high Km indicates weak binding, requiring higher substrate concentrations to reach Vmax.
Intrinsic Clearance
Intrinsic clearance measures the liver's ability to clear unbound drug without limitations to blood flow or binding considerations. It is defined as Vmax/Km. A drug that binds strongly to a metabolizing enzyme (small Km) has a high intrinsic clearance, while a drug that binds weakly (high Km) has a low intrinsic clearance. In drug-drug interactions, an increased Km for the victim drug results in a reduction of intrinsic clearance.
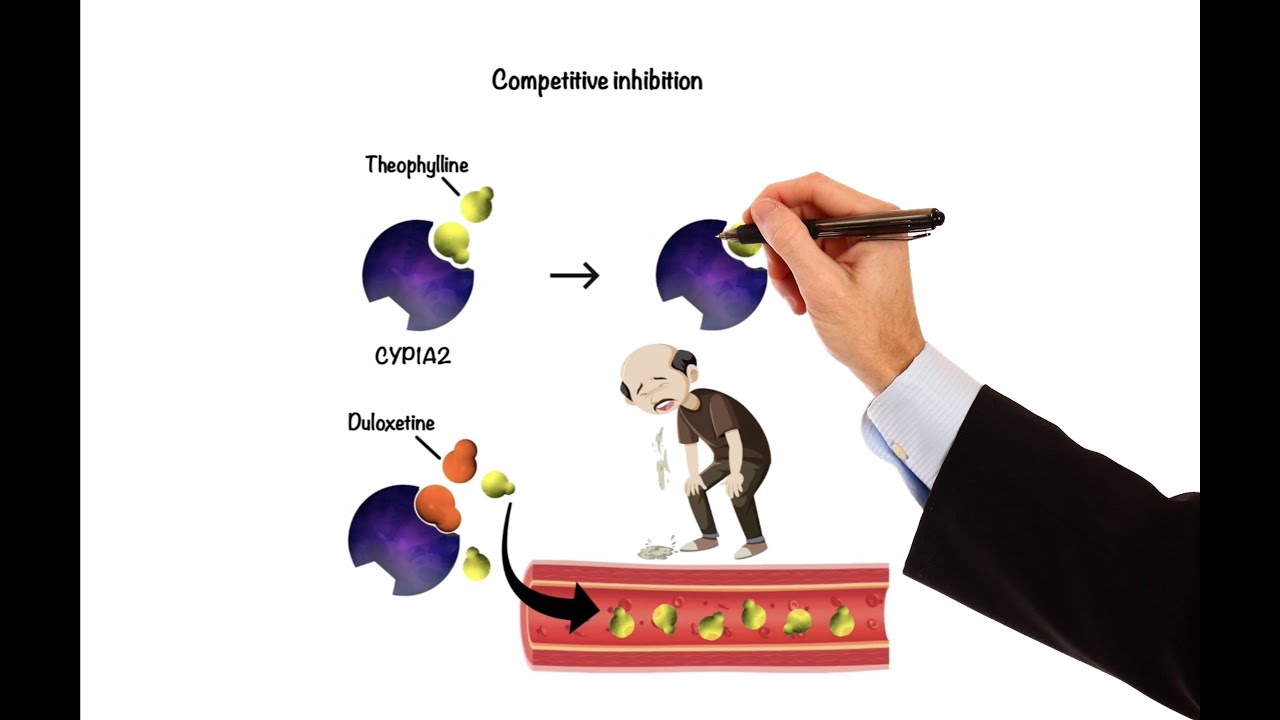
Competitive Inhibition
Competitive inhibition occurs when two substrates compete for the same active site on an enzyme, determined by their affinities and concentrations. A substrate with a stronger affinity (Drug A) can displace a weaker substrate (Drug B), leading to Drug B accumulation, increased Km, and reduced intrinsic clearance. For active drugs, this can increase plasma concentrations and adverse effects; for prodrugs, it can reduce the formation of the active metabolite, decreasing efficacy. To minimize competitive inhibition, competing substrates should be administered with as much time apart as possible. The effects of competitive inhibition are reversible and sensitive to substrate concentrations.
Non-Competitive Inhibition
Non-competitive inhibition involves an inhibitor binding to an allosteric site, causing a conformational change in the active site that prevents substrate binding. There is no direct competition at the active site, and this type of inhibition is typically long-lasting and cannot be overcome by increasing substrate concentration. Separating the time of dosing will not alleviate non-competitive inhibition. For example, Fluconazole binds to an allosteric site of CYP2C9, causing the active site to lose its affinity for Carvedilol, leading to increased serum concentrations of Carvedilol and increased risk of hypotension and bradycardia.
Mixed inhibition
Mixed inhibition occurs when an inhibitor can bind to either the enzyme's active site or allosteric site, preventing substrate binding. Mixed inhibitors are usually more potent than competitive or non-competitive inhibitors. For example, Ketoconazole, a mixed CYP3A inhibitor, can occupy either the active site or allosteric site of CYP3A, preventing the metabolism of Midazolam and leading to its accumulation in plasma, increasing the risk of side effects.
Irreversible Inhibition
Irreversible inhibition, also known as mechanism-based inhibition, includes alternate substrate inhibition and suicide inhibition. Alternate substrate inhibition occurs when a stable intermediate metabolite forms covalent bonds at the active site, creating a stable complex that is not easily broken. For example, Calrithromycin generates a nitrosoalkene intermediate that forms covalent bonds with the active site of CYP3A4. Suicide inhibition involves a highly reactive intermediate forming strong, irreversible covalent bonds with the enzyme, significantly changing its structure or destroying it. For example, Esomeprazole inhibits CYP2C19 by crosslinking heme and apoprotein moieties, altering its structure. This is problematic when co-administered with Clopidogrel, as it reduces the formation of Clopidogrel's active metabolite, increasing the risk of cardiovascular events.